
by Editor | Apr 14, 2016 | Menopause
In the same month I went from menstrual cramps to hot flashes overnight. By Sheryl E. Mendlinger, PhD In 1994, at the age of 43, I was diagnosed with stage 1 invasive breast cancer. At that time, treatment options were very limited, it was more a “one size fits...
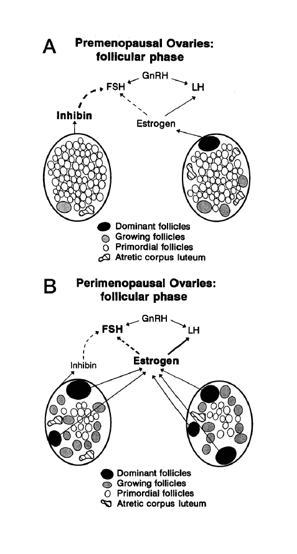
by Editor | Apr 5, 2016 | Health Care, Menopause, Perimenopause
Making sense of the many names for women’s reproductive aging by Dr. Jerilynn C. Prior Jerilynn C. Prior BA, MD, FRCPC, ABIM, ABEM is a Professor of Endocrinology and Metabolism at the University of British Columbia in Vancouver, B.C. She is the founder (2002) and...

by Heather Dillaway | Nov 8, 2012 | Communication, Internet, Media, Menopause, Menstruation
It turns out that phthalates – chemicals found in cosmetics, hairspray, packaged food, household cleaners, and other common plastic items – are causing early menopause. At least according to one new study that is getting a lot of hype in the past week or...
by Paula Derry | Oct 15, 2012 | Menopause, New Research
Many of us do our own health research, either because we have a specific question or simply to keep up with the news. If we don’t read the original scientific articles, we rely on experts to provide summaries in newspapers, magazines, or on a variety of websites. It...
by Chris Hitchcock | Nov 22, 2011 | Language, Menopause, New Research
Recently Heather Dillaway blogged about the challenges and frustrations of naming, and this blog continues with that theme, looking at a recent article about increased rates of “ovarian failure” following ovary-preserving hysterectomy. “Ovary-saving...





